
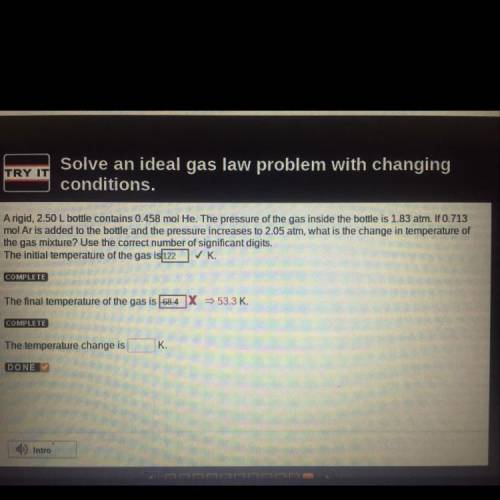
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm. If 0.713
mol Ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of
the gas mixture? Use the correct number of significant digits.
The temperature change is

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3
You know the right answer?
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm....
Questions




Mathematics, 31.10.2020 01:00


Biology, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00


Mathematics, 31.10.2020 01:00

Biology, 31.10.2020 01:00

Chemistry, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00


Biology, 31.10.2020 01:00


Business, 31.10.2020 01:00

Arts, 31.10.2020 01:00

Computers and Technology, 31.10.2020 01:00

Mathematics, 31.10.2020 01:00




