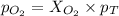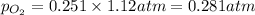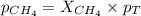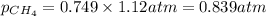Chemistry, 19.04.2020 00:48 st23pgardner
A mixture of 6.00 g of O2 (g) and 9.00 g of CH4 (g) is placed in a 15.0 L vessel at 0o C. What is the partial pressure of each gas? What is the total pressure in the vessel?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following molecules is polar? c3h7oh c2h5cooh
Answers: 1

Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1

Chemistry, 23.06.2019 09:30
Organisms that live in the alpine and taiga biomes have developed unique adaptations that aid in their survival. moss campion is one of the plants found in the alpine biome. it has small leaves and a cushion shape that protect it from the wind and freezing temperatures in the alpine. how has the moss campion adapted to enable its survival in the alpine biome? a. waxy needles b. cone-shaped c. thin trunks d. low-growing
Answers: 1
You know the right answer?
A mixture of 6.00 g of O2 (g) and 9.00 g of CH4 (g) is placed in a 15.0 L vessel at 0o C. What is th...
Questions


Chemistry, 27.10.2020 22:50



Mathematics, 27.10.2020 22:50

Mathematics, 27.10.2020 22:50

Spanish, 27.10.2020 22:50


Mathematics, 27.10.2020 22:50

Mathematics, 27.10.2020 22:50

Physics, 27.10.2020 22:50

Mathematics, 27.10.2020 22:50

Mathematics, 27.10.2020 22:50

Physics, 27.10.2020 22:50


Mathematics, 27.10.2020 22:50

Mathematics, 27.10.2020 22:50


Mathematics, 27.10.2020 22:50

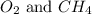 is, 0.281 atm and 0.839 atm respectively.
is, 0.281 atm and 0.839 atm respectively.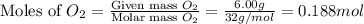
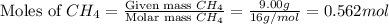
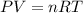
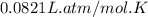
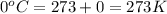
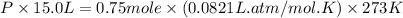
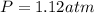
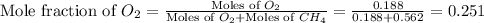
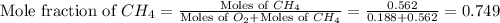
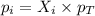
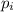 = partial pressure of gas
= partial pressure of gas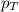 = total pressure of gas = 1.12 atm
= total pressure of gas = 1.12 atm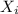 = mole fraction of gas
= mole fraction of gas