

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Use the following data to calculate the standard enthalpy of formation of pentane, C5H12(l)?
C...
C...
Questions

Social Studies, 01.12.2020 04:00

Computers and Technology, 01.12.2020 04:00

Mathematics, 01.12.2020 04:00


Mathematics, 01.12.2020 04:00

Chemistry, 01.12.2020 04:00





Mathematics, 01.12.2020 04:00

Social Studies, 01.12.2020 04:00






English, 01.12.2020 04:00

Biology, 01.12.2020 04:00

Social Studies, 01.12.2020 04:00


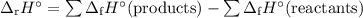
![\begin{array}{rcl}-3535.9 & = & [5\times(-393.5) -6 \times(-285.8)] - x\\-3535.9 & = & -3682.3 - x\\x& = & \textbf{-146.4 kJ/mol}\\\end{array}\\\text{The enthalpy of formation of pentane is } \large \boxed{\textbf{-146.4 kJ/mol}}](/tpl/images/0610/4927/daae3.png)


