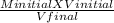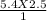Chemistry, 18.04.2020 10:00 keigleyhannah30
A2.5 L sample of a solution of sea water has a NaCl concentration of 5.4
M. What is the new concentration of this solution if it is boiled down to a
volume of 1.0 L?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5 m hcl? show all of the work needed to solve this problem. mg (s) + 2hcl (aq) → mgcl2 (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 01:50
7. what temperature is need to just dissolve 50 g of nh4cl in 75 g of water? '
Answers: 1

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
A2.5 L sample of a solution of sea water has a NaCl concentration of 5.4
M. What is the new co...
M. What is the new co...
Questions


Mathematics, 18.08.2019 15:30

Mathematics, 18.08.2019 15:30

English, 18.08.2019 15:30


Mathematics, 18.08.2019 15:30

Mathematics, 18.08.2019 15:30

Mathematics, 18.08.2019 15:30





Geography, 18.08.2019 15:30

Mathematics, 18.08.2019 15:30


Mathematics, 18.08.2019 15:30




History, 18.08.2019 15:30