-50100 J

Chemistry, 18.04.2020 01:04 rivera6681
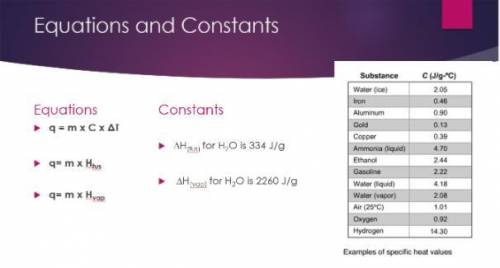
Calculate the amount of heat released to convert 150.0 g of to water to ice at 0ºC.
-50100 J
-339,000 J
-627 J
-307.5 J

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
Calculate the amount of heat released to convert 150.0 g of to water to ice at 0ºC.
-50100 J
-50100 J
Questions

Mathematics, 08.12.2020 21:40





Chemistry, 08.12.2020 21:40



Biology, 08.12.2020 21:40


Computers and Technology, 08.12.2020 21:40


Mathematics, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40

History, 08.12.2020 21:40



History, 08.12.2020 21:40

Mathematics, 08.12.2020 21:40

World Languages, 08.12.2020 21:40



