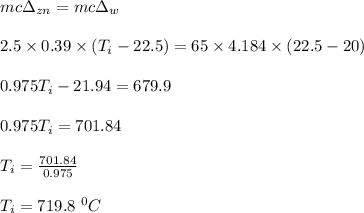A 2.50 g sample of zinc is heated, and then placed in a calorimeter containing 65.0 g of water. Temperature of water increases from 20.00 oC to 22.50 oC. The specific heat of zinc is 0.390 J/g oC. Assuming no heat loss, what was the initial temperature of the zinc metal sample?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
A 2.50 g sample of zinc is heated, and then placed in a calorimeter containing 65.0 g of water. Temp...
Questions

English, 25.10.2021 19:00


Mathematics, 25.10.2021 19:00

Advanced Placement (AP), 25.10.2021 19:00

Mathematics, 25.10.2021 19:00

Mathematics, 25.10.2021 19:00

History, 25.10.2021 19:00


Mathematics, 25.10.2021 19:00

English, 25.10.2021 19:00

Chemistry, 25.10.2021 19:00


English, 25.10.2021 19:00

English, 25.10.2021 19:00



Advanced Placement (AP), 25.10.2021 19:00

Social Studies, 25.10.2021 19:00

Chemistry, 25.10.2021 19:00

Business, 25.10.2021 19:00