
Chemistry, 02.10.2019 12:00 janahiac09
An equilibrium mixture of co, o2 and co2 at a certain temperature contains 0.0010 m co2 and 0.0100 m o2. at this temperature, kc equals 1.4 × 102 for the reaction: 2 co(g) + o2(g)⇌2 co2(g). what is the equilibrium concentration of co?
a) 7.1 × 10-7 m.
b) 8.4 × 10-4 m.
c) 1.4 × 10-2 m.
d) 1.2 × 10-1 m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 15:30
The gulf stream is a warm water current that flows away from the equator to northern europe. witch of these does it cause. a. crashes of warm and cool water in the ocean b.colder climates near the equator c.large waves on the cost of europe d.warm climates in northern europe
Answers: 1
You know the right answer?
An equilibrium mixture of co, o2 and co2 at a certain temperature contains 0.0010 m co2 and 0.0100 m...
Questions






History, 02.08.2019 22:00



History, 02.08.2019 22:00



Mathematics, 02.08.2019 22:00

Biology, 02.08.2019 22:00


Arts, 02.08.2019 22:00


Mathematics, 02.08.2019 22:00

Mathematics, 02.08.2019 22:00


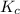
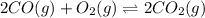
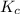 for above reaction is:
for above reaction is:![K_c=\frac{[CO_2]^2}{[CO]^2[O_2]}](/tpl/images/0282/9569/3f046.png)
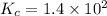
![[CO_2]=0.0010M](/tpl/images/0282/9569/bba46.png)
![[O_2]=0.0100M](/tpl/images/0282/9569/e6846.png)
![1.4\times 10^2=\frac{(0.0010)^2}{[CO]^2\times (0.0100)}](/tpl/images/0282/9569/4f227.png)
![[CO]=\sqrt{\frac{(0.0010)^2}{0.0100\times 1.4\times 10^2}}](/tpl/images/0282/9569/54368.png)
![[CO]=8.4\times 10^{-4}M](/tpl/images/0282/9569/712c0.png)


