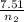A flexible container at an initial volume of 8.15 L contains 7.51 mol of a gas. More gas is
th...

Chemistry, 16.04.2020 23:03 Arianaagosto
A flexible container at an initial volume of 8.15 L contains 7.51 mol of a gas. More gas is
then added to the container until it reaches a final volume of 13.9 L. Assuming the
pressure and the temperature of the gas remained constant, calculate the moles of gas
added to the container.
15.1 mol
O 5.5 mol
0 4.40 mol
12.8 mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

You know the right answer?
Questions

History, 05.05.2021 22:00

Mathematics, 05.05.2021 22:00

English, 05.05.2021 22:00

Chemistry, 05.05.2021 22:00


Mathematics, 05.05.2021 22:00

Advanced Placement (AP), 05.05.2021 22:00


History, 05.05.2021 22:00

Chemistry, 05.05.2021 22:00




Mathematics, 05.05.2021 22:00

Social Studies, 05.05.2021 22:00

Mathematics, 05.05.2021 22:00




Mathematics, 05.05.2021 22:00

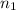 ) = 7.51 moles
) = 7.51 moles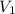 ) = 8.15 L
) = 8.15 L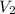 ) = 13.9 L
) = 13.9 L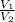 =
= 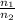
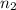 represents the final moles
represents the final moles 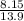 =
=