pressure of the gas?

Chemistry, 16.04.2020 23:15 cassiegagnier73
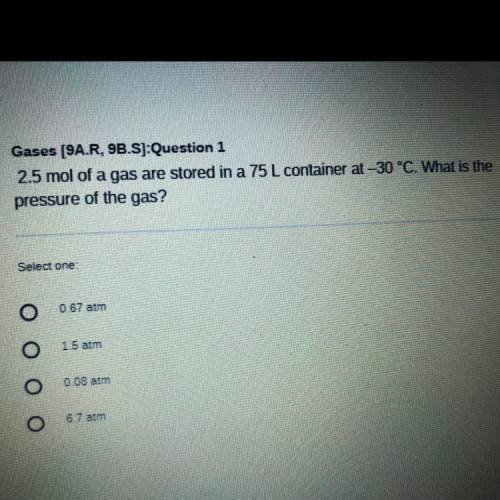
2.5 mol of a gas are stored in a 75 L container at -30 °C. What is the
pressure of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
2.5 mol of a gas are stored in a 75 L container at -30 °C. What is the
pressure of the gas?
pressure of the gas?
Questions





Mathematics, 25.05.2020 01:57

Mathematics, 25.05.2020 01:57

Biology, 25.05.2020 01:57










Physics, 25.05.2020 01:57





