Chemistry, 16.04.2020 22:09 zenaidazurita1p6bs1d
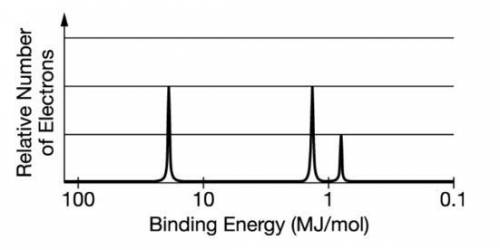
The photoelectron spectrum for the element boron is represented above. Which of the following best explains how the spectrum is consistent with the electron shell model of the atom? The spectrum shows an odd number electrons. A The spectrum shows a single electron in the 2p subshell. B The spectrum shows equal numbers of electrons in the first and second electron shells. C The spectrum shows three electrons with the same binding energy in the second electron shell.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
State the formula for density in words and mathematical symbols
Answers: 2

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
The photoelectron spectrum for the element boron is represented above. Which of the following best e...
Questions

Mathematics, 21.02.2021 06:00

Mathematics, 21.02.2021 06:00

Mathematics, 21.02.2021 06:00



English, 21.02.2021 06:00

Mathematics, 21.02.2021 06:00



Geography, 21.02.2021 06:00

Physics, 21.02.2021 06:00



Mathematics, 21.02.2021 06:00




Mathematics, 21.02.2021 06:10


History, 21.02.2021 06:10