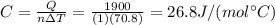A coffee cup (or constant pressure) calorimeter contains 108.0 g of water at an initial temperature of 25.0°C. 118.7 g of tin metal at a temperature of 100°C is added. The final temperature in the calorimeter is 29.2°C. What is the molar heat capacity of the tin? The molar heat capacity of water is 75.4 J / (mol•°C). Assume that the heat capacity of the coffee cup is negligible.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

You know the right answer?
A coffee cup (or constant pressure) calorimeter contains 108.0 g of water at an initial temperature...
Questions

Mathematics, 31.01.2020 14:55

English, 31.01.2020 14:55


Mathematics, 31.01.2020 14:55

Biology, 31.01.2020 14:55

Mathematics, 31.01.2020 14:55

English, 31.01.2020 14:55

Mathematics, 31.01.2020 14:55



Mathematics, 31.01.2020 14:55



History, 31.01.2020 14:55

Chemistry, 31.01.2020 14:55

Mathematics, 31.01.2020 14:55


Geography, 31.01.2020 14:56


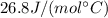
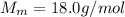
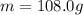
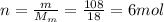
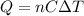
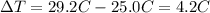 is the change in temperature of the water
is the change in temperature of the water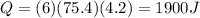
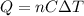
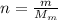 is the number of moles of tin, where
is the number of moles of tin, where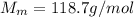 is the molar mass of tin
is the molar mass of tin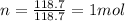
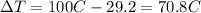 is the change in temperature of the tin
is the change in temperature of the tin