
Chemistry, 16.04.2020 20:01 Jerryholloway5871
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
2NaN3(s)-> 2Na(s) + 3N2(s)
An unknown amount of sodium azide (NaN3) reacts and produces 744 mL of nitrogen gas. What mass of sodium metal is produced?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
...
...
Questions


Biology, 01.10.2019 06:00

English, 01.10.2019 06:00

Mathematics, 01.10.2019 06:00



Chemistry, 01.10.2019 06:00


Computers and Technology, 01.10.2019 06:00



Mathematics, 01.10.2019 06:00


Biology, 01.10.2019 06:00



English, 01.10.2019 06:00



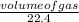
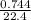 = 0.03moles
= 0.03moles 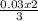 = 0.02mole
= 0.02mole

