

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2


Chemistry, 22.06.2019 20:00
For the reaction c6h14(g) & longrightarrow; c6h6(g) + 4h2(g), δp(h2)/δt was found to be 2.5 x 10-2 atm/s, where δp(h2) is the change in pressure of hydrogen. determine δp(c6h14)/δt for this reaction at the same time.
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Determine the total pressure of gas mixture the contains oxygen, nitrogen, and helium if the partial...
Questions

Biology, 15.01.2021 01:00

Mathematics, 15.01.2021 01:00



Chemistry, 15.01.2021 01:00

Advanced Placement (AP), 15.01.2021 01:00

English, 15.01.2021 01:00


Chemistry, 15.01.2021 01:00

Mathematics, 15.01.2021 01:00

Mathematics, 15.01.2021 01:00

Mathematics, 15.01.2021 01:00


History, 15.01.2021 01:00


Mathematics, 15.01.2021 01:00


Advanced Placement (AP), 15.01.2021 01:00


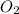 = 0.197 atm
= 0.197 atm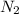 = 0.461 atm
= 0.461 atm


