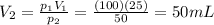Chemistry, 16.04.2020 09:35 emmaguentherp3hjd3
A can of coke contains 25 mL of carbon dioxide gas at 100kPa. If you take it on a hike up Mount Everest and the pressure decreases to 50 kPa, what will the new volume of the carbon dioxide gas in your coke can be?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3



Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
A can of coke contains 25 mL of carbon dioxide gas at 100kPa. If you take it on a hike up Mount Ever...
Questions

Mathematics, 26.03.2021 22:30

Mathematics, 26.03.2021 22:30


Mathematics, 26.03.2021 22:30

Mathematics, 26.03.2021 22:30

History, 26.03.2021 22:30



Mathematics, 26.03.2021 22:30


History, 26.03.2021 22:30

Biology, 26.03.2021 22:30

Mathematics, 26.03.2021 22:30


Mathematics, 26.03.2021 22:30

History, 26.03.2021 22:30



Mathematics, 26.03.2021 22:30

Mathematics, 26.03.2021 22:30

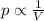
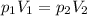
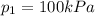 is the initial pressure of the gas in the coke
is the initial pressure of the gas in the coke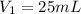 is the initial volume
is the initial volume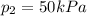 is the final pressure
is the final pressure