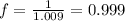Chemistry, 16.04.2020 04:57 pleasedontspamme
You prepare a buffer solution from 10.0 mL of 0.100 M MOPS (3‑morpholinopropane‑1‑sulfonic acid) and 10.0 mL of 0.074 M NaOH . 0.074 M NaOH. Next, you add 1.00 mL of 3.51 × 10 − 5 M 3.51×10−5 M lidocaine to this mixture. Denoting lidocaine as L, calculate the fraction of lidocaine present in the form LH +

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
You prepare a buffer solution from 10.0 mL of 0.100 M MOPS (3‑morpholinopropane‑1‑sulfonic acid) and...
Questions




Computers and Technology, 24.02.2020 16:55







English, 24.02.2020 16:55

Mathematics, 24.02.2020 16:55








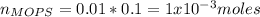
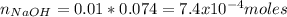

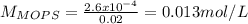
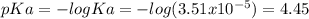
![pH=pKa+log\frac{[NaOH]}{[MOPS]} =4.45+log\frac{0.037}{0.013} =4.9](/tpl/images/0604/7273/c3441.png)
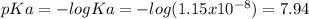
![pH=pKa+log\frac{[base]}{[acid]} \\4.9=7.94+log\frac{[base]}{[acid]}\\log\frac{[base]}{[acid]}=-3.04\\base/acid=9.12x10^{-4}](/tpl/images/0604/7273/6eb33.png)