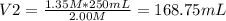You need to prepare 250.0 mL of a 1.35 M HCl solution from a 2.00 M HCl stock solution.
...

Chemistry, 16.04.2020 04:56 Bjehnsen3720
You need to prepare 250.0 mL of a 1.35 M HCl solution from a 2.00 M HCl stock solution.
a. Which glassware should you use to make the solution?
A. beaker
B. Erlenmeyer flask
C. volumetric flask
b. How should the correct amount of stock be obtained?
A. Measure out x g on a balance
B. Measure out x mL using a volumetric pipet
C. Measure out x mL using a graduated cylinder
c. Based on your answer above, what is the value of x?
d. How should the solution be mixed together?
A. Fill the container to the 250 mL mark then add the correct amount of stock solution.
B. Add the correct amount of stock solution then fill to the 250 mL mark with water.
C. Fill the container partially with water, add the correct amount of stock solution, then fill to the 250 mL mark with water.
D. None of these is the correct way to mix the stock solution.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 06:10
How much would the freezing point of water decrease if 4 mol of nacl were added to 1 kg of water (kf=1.86 degrees c/(mol/kg) for water and i=2 for nacl a- 7.44 degrees c b- 14.88 c 3.72 d 1.86
Answers: 1
You know the right answer?
Questions


Mathematics, 06.05.2020 22:02

Mathematics, 06.05.2020 22:02

Mathematics, 06.05.2020 22:02




Mathematics, 06.05.2020 22:02

English, 06.05.2020 22:02

Mathematics, 06.05.2020 22:02

Mathematics, 06.05.2020 22:02


English, 06.05.2020 22:02





History, 06.05.2020 22:02

Medicine, 06.05.2020 22:02

English, 06.05.2020 22:02