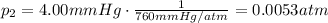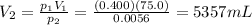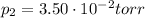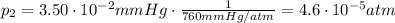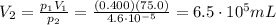Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
3. A sample of krypton gas occupies 75.0 mL at 0.400 atm. If the temperature remained constant, what...
Questions




















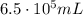
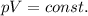
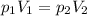
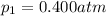 is the initial pressure
is the initial pressure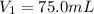 is the initial volume
is the initial volume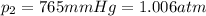 is the final pressure (using the conversion factor
is the final pressure (using the conversion factor 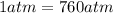 )
)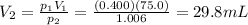
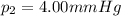 is the final pressure
is the final pressure