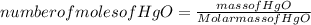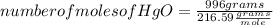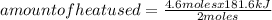Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 18:50
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3
You know the right answer?
A mercury mirror forms inside a test tube as a result of the thermal decomposition of mercury(ii) ox...
Questions

Mathematics, 25.03.2021 17:00




Biology, 25.03.2021 17:00

Mathematics, 25.03.2021 17:00

Mathematics, 25.03.2021 17:00

Mathematics, 25.03.2021 17:00

Mathematics, 25.03.2021 17:00


Mathematics, 25.03.2021 17:00


Mathematics, 25.03.2021 17:00


Computers and Technology, 25.03.2021 17:00


Mathematics, 25.03.2021 17:00

English, 25.03.2021 17:00


Mathematics, 25.03.2021 17:00