
Suppose you add 0.2469 g of PbCl2(s) to 50.0 mL of water. In the resulting saturated solution, you find that the concentration of PbX2+(aq) is 0.0159 M and the concentration of C l − ( a q ) ClX−(aq) is 0.0318 M. What is the value of the equilibrium constant, Ksp, for the dissolution of PbCl2?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
How air particles exert a pressure on the inside of the balloon
Answers: 1

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3
You know the right answer?
Suppose you add 0.2469 g of PbCl2(s) to 50.0 mL of water. In the resulting saturated solution, you f...
Questions








History, 18.10.2020 14:01



Biology, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01

Mathematics, 18.10.2020 14:01


History, 18.10.2020 14:01

English, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Social Studies, 18.10.2020 14:01

Chemistry, 18.10.2020 14:01

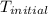 = a - -
= a - -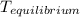 = a - s s 2s
= a - s s 2s

