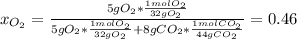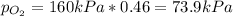Chemistry, 16.04.2020 01:01 genyjoannerubiera
A tank contains an ideal gas mixture of 5 g of O2 and 8 g of CO2 at 160kPa and specified temperature. If O2 were separated from the mixture and stored at mixture temperature and in the same tank, its pressure would be, in kPa (round to nearest integer; for example if the answer is 14.6kPa, write 15; if the answer is 14.49L write 14; do not include the units in your answer

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 21.06.2019 17:00
What were the success and failures that came to boyle’s excitements?
Answers: 1

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2
You know the right answer?
A tank contains an ideal gas mixture of 5 g of O2 and 8 g of CO2 at 160kPa and specified temperature...
Questions


Mathematics, 26.10.2020 20:40

History, 26.10.2020 20:40


Mathematics, 26.10.2020 20:40

Health, 26.10.2020 20:40


Biology, 26.10.2020 20:40


History, 26.10.2020 20:40


Mathematics, 26.10.2020 20:40




Computers and Technology, 26.10.2020 20:40



Mathematics, 26.10.2020 20:40