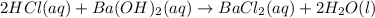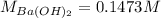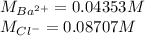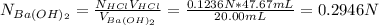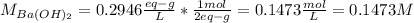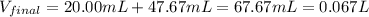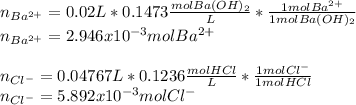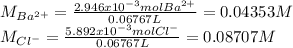A 20.00 mL Ba(OH)_2 solution of unknown concentration was neutralized by the addition of 47.67 mL of a 0.1236 M HCl solution. Write the balanced molecular equation for the neutralization reaction between HCl and Ba(OH)_2 in aqueous solution. Include physical states. 2HCl(aq) + Ba(OH)_2(aq) rightarrow BaCl_2(aq) + 2H_2O(l) Calculate the concentration of Ba(OH)_2 in the original 20.00 mL solution Calculate the concentrations of Ba^2+ and Cl^- in solution following the neutralization reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
A 20.00 mL Ba(OH)_2 solution of unknown concentration was neutralized by the addition of 47.67 mL of...
Questions


Mathematics, 11.03.2021 08:00

Social Studies, 11.03.2021 08:00


Mathematics, 11.03.2021 08:00

History, 11.03.2021 08:00



Mathematics, 11.03.2021 08:00


Mathematics, 11.03.2021 08:00


History, 11.03.2021 08:00

Mathematics, 11.03.2021 08:00

Mathematics, 11.03.2021 08:00


Mathematics, 11.03.2021 08:00

Mathematics, 11.03.2021 08:00