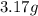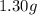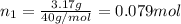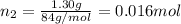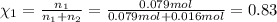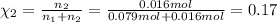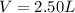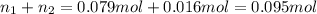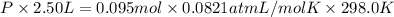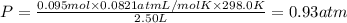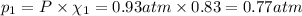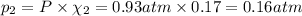Chemistry, 15.04.2020 22:05 cjjjjjjjjjjjjj
If a gaseous mixture is made by combining 3.17 g Ar 3.17 g Ar and 1.30 g Kr 1.30 g Kr in an evacuated 2.50 L container at 25.0 ∘ C, 25.0 ∘C, what are the partial pressures of each gas, P Ar PAr and P Kr , PKr, and what is the total pressure, P total , Ptotal, exerted by the gaseous mixture?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
You know the right answer?
If a gaseous mixture is made by combining 3.17 g Ar 3.17 g Ar and 1.30 g Kr 1.30 g Kr in an evacuate...
Questions

Mathematics, 30.04.2021 21:10


English, 30.04.2021 21:10


Chemistry, 30.04.2021 21:10

Mathematics, 30.04.2021 21:10

English, 30.04.2021 21:10

Computers and Technology, 30.04.2021 21:10

English, 30.04.2021 21:10



Biology, 30.04.2021 21:10


History, 30.04.2021 21:10




Mathematics, 30.04.2021 21:10

Mathematics, 30.04.2021 21:10