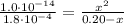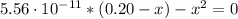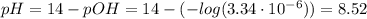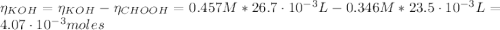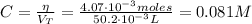Chemistry, 16.04.2020 00:36 RoxanneDuartee
Determine the pH during the titration of 23.5 mL of 0.346 M formic acid (Ka = 1.8×10-4) by 0.457 M KOH at the following points.
(a) Before the addition of any KOH
(b) After the addition of 4.00 mL of KOH
(c) At the half-equivalence point (the titration midpoint)
(d) At the equivalence point
(e) After the addition of 26.7 mL of KOH

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2


You know the right answer?
Determine the pH during the titration of 23.5 mL of 0.346 M formic acid (Ka = 1.8×10-4) by 0.457 M K...
Questions

Chemistry, 07.12.2020 22:00


Health, 07.12.2020 22:00



Biology, 07.12.2020 22:00


English, 07.12.2020 22:00


Mathematics, 07.12.2020 22:00

History, 07.12.2020 22:00





History, 07.12.2020 22:00


History, 07.12.2020 22:00


![pH = -log([H_{3}O^{+}])](/tpl/images/0603/8843/6ab72.png) (1)
(1) ![K_{a} = \frac{[CHOO^{-}][H_{3}O^{+}]}{[CHOOH]}](/tpl/images/0603/8843/c4333.png) (3)
(3)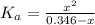
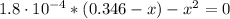 (4)
(4)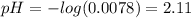
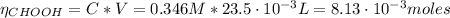
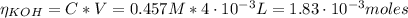
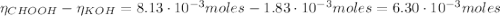
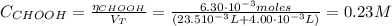
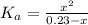
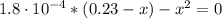
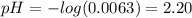
![pH = pKa + log(\frac{[CHOO^{-}]}{[CHOOH]})](/tpl/images/0603/8843/112dd.png)
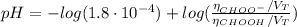
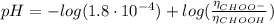
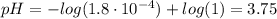
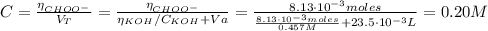
![K_{b} = \frac{[CHOOH][OH^{-}]}{[CHOO^{-}]}](/tpl/images/0603/8843/a45f9.png)
![\frac{K_{w}}{K_{a}} = \frac{[CHOOH][OH^{-}]}{[CHOO^{-}]}](/tpl/images/0603/8843/7611b.png)