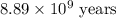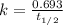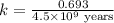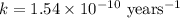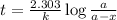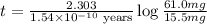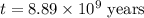Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
The half-life for the process 238u→206pb is 4.5×109 yr. a mineral sample contains 61.0 mg of 238u an...
Questions


Mathematics, 27.10.2019 21:43

Social Studies, 27.10.2019 21:43

Mathematics, 27.10.2019 21:43



Mathematics, 27.10.2019 21:43




Social Studies, 27.10.2019 21:43

Mathematics, 27.10.2019 21:43


Business, 27.10.2019 21:43


English, 27.10.2019 21:43

Health, 27.10.2019 21:43


World Languages, 27.10.2019 21:43

Mathematics, 27.10.2019 21:43