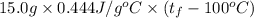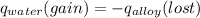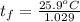Chemistry, 15.04.2020 20:48 jforeman42
A 15.0 g sample of nickel metal is heated to 100.0 degrees C and dropped into 55.0 g of water, initially at 23.0 degrees C. Assuming that all the heat lost by nickel is absorbed by the water, calculate the final temperature of the nickel and water. (C

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:30
Voltaic cells produce a positive overall charge. what does this indicate? a. the reaction is likely to be endothermic. b. the reaction is spontaneous. c. the reaction is not likely to occur. d. the reaction is not spontaneous.
Answers: 3

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

You know the right answer?
A 15.0 g sample of nickel metal is heated to 100.0 degrees C and dropped into 55.0 g of water, initi...
Questions




Social Studies, 31.03.2021 01:30



Biology, 31.03.2021 01:30

Mathematics, 31.03.2021 01:30




Mathematics, 31.03.2021 01:30

Mathematics, 31.03.2021 01:30







 .
.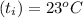 ,
, 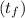 = ?,
= ?,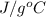 ,
, 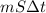
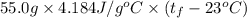
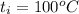 ,
,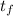 = ?
= ?