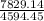Chemistry, 15.04.2020 17:17 kelseatuttleni
A mass of 35.0 g of an unknown solid initially at 170.0 ∘C is added to an ideal constant-pressure calorimeter containing 100.0 g of water (Cs, water=4.184 J/(g⋅∘C)) initially at 20.0 ∘C. After the mixture reaches thermal equilibrium, the final temperature is recorded to be 38.73 ∘C. What is the specific heat capacity of the unknown solid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1


Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3
You know the right answer?
A mass of 35.0 g of an unknown solid initially at 170.0 ∘C is added to an ideal constant-pressure ca...
Questions












Computers and Technology, 21.11.2019 22:31




English, 21.11.2019 22:31