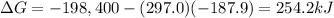For the process 2SO2(g) + O2(g) --> 2SO3(g),
ΔS = –187.9 J/K and ΔH = –198.4 kJ at 29...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 03:30
Explain why pure hydrogen cyanide does not conduct electricity, but become a conductor when it is dissolved in water? (at room temp, pure hcn exists as a volatile liquid)
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Questions

Geography, 14.07.2019 16:00

Arts, 14.07.2019 16:00






Chemistry, 14.07.2019 16:00

Mathematics, 14.07.2019 16:00

Mathematics, 14.07.2019 16:00

Health, 14.07.2019 16:00


Mathematics, 14.07.2019 16:00




Mathematics, 14.07.2019 16:00

History, 14.07.2019 16:00

Mathematics, 14.07.2019 16:00

Mathematics, 14.07.2019 16:00

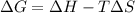
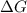 is the Gibbs free energy
is the Gibbs free energy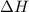 is the change in enthalpy of the reaction
is the change in enthalpy of the reaction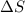 is the change in entropy
is the change in entropy