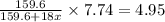Chemistry, 15.04.2020 04:41 Madisonk3571
A hydrated form of copper sulfate ( CuSO 4 ⋅ x H 2 O ) is heated to drive off all of the water. If there is initially 7.74 g of hydrated salt and there is 4.95 g of anhydrous CuSO 4 after heating, find the number of water molecules associated with each CuSO 4 formula unit.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
You know the right answer?
A hydrated form of copper sulfate ( CuSO 4 ⋅ x H 2 O ) is heated to drive off all of the water. If t...
Questions

Biology, 02.02.2021 16:00

Mathematics, 02.02.2021 16:00



Mathematics, 02.02.2021 16:00


Mathematics, 02.02.2021 16:00

Mathematics, 02.02.2021 16:00


Mathematics, 02.02.2021 16:00

Mathematics, 02.02.2021 16:00

Mathematics, 02.02.2021 16:00

Social Studies, 02.02.2021 16:00

Mathematics, 02.02.2021 16:00

Mathematics, 02.02.2021 16:00





Biology, 02.02.2021 16:00

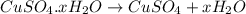
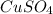 = 159.6 g/mol
= 159.6 g/mol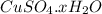 decomposes to give = 159.6g of
decomposes to give = 159.6g of 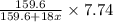 g
g