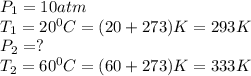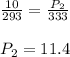A dive tank is designed and built to be safe if the difference between the pressure inside the tank and the pressure outside the tank does not exceed 12 atm. A diver has the tank filled at the dive store at a temperature of 20o C to a pressure of 10 atm. The diver places the tank into his car and parks the car in the sun where the temperature of the gas in the tank increases to 60o C. Is the tank safe at this temperature? You must show the setup of an equation, the final answer with units , and a short statement whether the tank is safe or not.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
A dive tank is designed and built to be safe if the difference between the pressure inside the tank...
Questions

Mathematics, 27.02.2021 19:10



Mathematics, 27.02.2021 19:10

Social Studies, 27.02.2021 19:10


Mathematics, 27.02.2021 19:10


Spanish, 27.02.2021 19:10

Social Studies, 27.02.2021 19:10



History, 27.02.2021 19:10


Chemistry, 27.02.2021 19:10

Mathematics, 27.02.2021 19:10


Biology, 27.02.2021 19:10

Biology, 27.02.2021 19:10

Physics, 27.02.2021 19:10

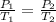
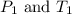 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.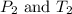 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.