
Chemistry, 29.08.2019 22:40 austintules2005
How many moles of ethylene (c2h4) can react with 12.9 liters of oxygen gas at 1.2 atmospheres and 297 kelvin?
c2h4(g) + 3o2(g) yields 2co2(g) + 2h2o(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2


Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
How many moles of ethylene (c2h4) can react with 12.9 liters of oxygen gas at 1.2 atmospheres and 29...
Questions

Biology, 24.08.2019 23:30


Mathematics, 24.08.2019 23:30


Business, 24.08.2019 23:30


Biology, 24.08.2019 23:30


Physics, 24.08.2019 23:30

Health, 24.08.2019 23:30



Mathematics, 24.08.2019 23:30

Biology, 24.08.2019 23:30


Mathematics, 24.08.2019 23:30


English, 24.08.2019 23:30


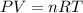
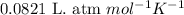

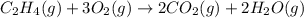
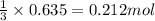 of ethylene gas
of ethylene gas

