
Carbon's valence of four most directly results from its a. tetrahedral shape. b. four electrons in the valence shell that can form four covalent bonds. c. ability to form single, double, and triple bonds. d. ability to form chains and rings of carbon atoms. 2. Hydrocarbons are not soluble in water because a. they are hydrophilic. b. their C-H bonds are nonpolar. c. they do not ionize. d. they are lighter than water. 3. Which of the following is not true of an asymmet- ric carbon atom

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
Carbon's valence of four most directly results from its a. tetrahedral shape. b. four electrons in t...
Questions



Social Studies, 30.11.2021 23:30





Business, 30.11.2021 23:30







Mathematics, 30.11.2021 23:30


Chemistry, 30.11.2021 23:30

Advanced Placement (AP), 30.11.2021 23:30

Business, 30.11.2021 23:30

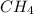 is a hydrocarbon.
is a hydrocarbon.


