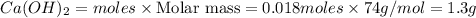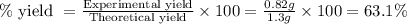Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
...

Chemistry, 15.04.2020 03:36 Crtive5515
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:
CaO(s)+H2O(l)→Ca(OH)2(s)
In a particular experiment, a 1.00-g sample of CaO is reacted with excess water and 0.82 g of Ca(OH)2 is recovered. What is the percent yield in this experiment?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 23.06.2019 01:30
In which phase of mitosis do the spindle fibers pull the chromosomes apart to opposite sides of the cell ?
Answers: 1

Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Questions

Mathematics, 13.01.2021 14:00




Mathematics, 13.01.2021 14:00

Social Studies, 13.01.2021 14:00



Social Studies, 13.01.2021 14:00


Medicine, 13.01.2021 14:00









History, 13.01.2021 14:00

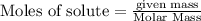
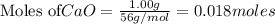
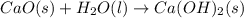
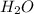 is the excess reagent,
is the excess reagent, 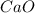 acts as the limiting reagent and it limits the formation of product.
acts as the limiting reagent and it limits the formation of product.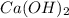
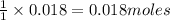 of
of