Chemistry, 15.04.2020 03:34 alekvtaylor
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution containing sodium iodide: 2NaI(aq) +Cl2(g) → I2(s) +2NaCl(aq) How many grams of iodide, NaI, must be used to produce 55.6 g of iodine, I2?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 02:10
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2
You know the right answer?
Iodine is prepared both in the laboratory and commercially by adding Cl2(g) to an aqueous solution c...
Questions

Mathematics, 30.06.2021 19:30

Mathematics, 30.06.2021 19:30

Mathematics, 30.06.2021 19:30

Mathematics, 30.06.2021 19:30





Mathematics, 30.06.2021 19:30




Chemistry, 30.06.2021 19:30



Mathematics, 30.06.2021 19:30




Spanish, 30.06.2021 19:30

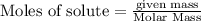
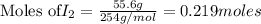
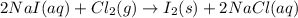
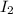 are produced by = 2 moles of
are produced by = 2 moles of 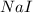
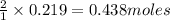 of
of