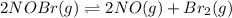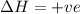Chemistry, 15.04.2020 03:13 joeykyle05
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reaction is endothermic. What do you expect to happen to the concentration of NO if the temperature is doubled? 2NOBr(g) ⇌ 2NO(g) + Br2(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
For the following reaction, the equilibrium constant Kc is 2.0 at a certain temperature. The reactio...
Questions


Mathematics, 17.06.2021 02:30

History, 17.06.2021 02:30

Mathematics, 17.06.2021 02:30

Mathematics, 17.06.2021 02:40


History, 17.06.2021 02:40


Mathematics, 17.06.2021 02:40


Biology, 17.06.2021 02:40



Mathematics, 17.06.2021 02:40

Social Studies, 17.06.2021 02:40

Mathematics, 17.06.2021 02:40

Chemistry, 17.06.2021 02:40


Social Studies, 17.06.2021 02:40