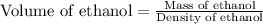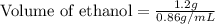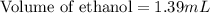Chemistry, 15.04.2020 02:56 Blazingangelkl
Recrystallization Step: The procedure asks for using equal amount by mass of the crude product and the recrystallization solvent, 95% ethanol. Suppose you use 1.2g of product, how many mL of 95% ethanol would you add? Write the answer as a number (without units) with 3 significant figures (e. g. 2.15). Density of 95% ethanol = 0.86 g/mL

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1

Chemistry, 22.06.2019 18:10
Given the following at 25c calculate delta hf for hcn (g) at 25c. 2nh3 (g) +3o2 (g) + 2ch4 (g) > 2hcn (g) + 6h2o (g) delta h rxn= -870.8 kj. delta hf=-80.3 kj/mol for nh3 (g), -74.6 kj/mol for ch4, and -241.8 kj/mol for h2o (g)
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
Recrystallization Step: The procedure asks for using equal amount by mass of the crude product and t...
Questions


Mathematics, 05.12.2020 01:00

Computers and Technology, 05.12.2020 01:00

Chemistry, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00


Mathematics, 05.12.2020 01:00



Biology, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00

English, 05.12.2020 01:00



English, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00

Mathematics, 05.12.2020 01:00