
Chemistry, 15.04.2020 02:55 bvbbridesmaid5519
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At standard conditions, 112 mL of the gaseous compound weighs 0.21 g. What is the molecular formula for the compound

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1


You know the right answer?
Analysis of a sample of a gaseous compound shows that it contains 85.7% C and 14.3% H by mass. At st...
Questions



Computers and Technology, 27.10.2019 00:43

Mathematics, 27.10.2019 00:43

Mathematics, 27.10.2019 00:43







Health, 27.10.2019 00:43

Physics, 27.10.2019 00:43

History, 27.10.2019 00:43




Chemistry, 27.10.2019 00:43


History, 27.10.2019 00:43







 = 1(12) + 2(1) = 14 g/eq.
= 1(12) + 2(1) = 14 g/eq.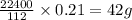 of compound
of compound




