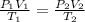Chemistry, 15.04.2020 02:54 laladance123
An ideal gas is contained in a cylinder with a volume of 500 mL at a temperature of 30.0 C and a pressure of 710 torr. The gas is then compressed to a volume of 25 mL, and the temperature is raised to 820 C. What is the new pressure of the gas

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
An ideal gas is contained in a cylinder with a volume of 500 mL at a temperature of 30.0 C and a pre...
Questions

History, 28.12.2019 18:31

Advanced Placement (AP), 28.12.2019 18:31

History, 28.12.2019 18:31

Computers and Technology, 28.12.2019 18:31

Social Studies, 28.12.2019 18:31




Biology, 28.12.2019 18:31

Mathematics, 28.12.2019 18:31

Geography, 28.12.2019 18:31

Physics, 28.12.2019 18:31

Mathematics, 28.12.2019 18:31



Mathematics, 28.12.2019 18:31


Mathematics, 28.12.2019 18:31


Chemistry, 28.12.2019 18:31