
5.00 grams of sodium hydroxide was placed in 100.0 grams of water in a coffee cup calorimeter. The temperature of the water increased from 25.0 deg C to 35.2 deg C. Find the heat of solution (ΔHsoln) for sodium hydroxide in kJ/mol. (Specific heat of water = 4.184J/g degC)?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3
You know the right answer?
5.00 grams of sodium hydroxide was placed in 100.0 grams of water in a coffee cup calorimeter. The t...
Questions

Computers and Technology, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01




Computers and Technology, 20.09.2020 16:01


Mathematics, 20.09.2020 16:01



Computers and Technology, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01


Mathematics, 20.09.2020 16:01

Chemistry, 20.09.2020 16:01





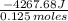
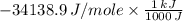 = -34.139 kJ/mol
= -34.139 kJ/mol


