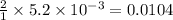A chemist prepares a solution of magnesium chloride MgCl2 by measuring out 49.mg of MgCl2 into a 100.mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl−anions in the chemist's solution. Be sure your answer is rounded to the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 23.06.2019 03:30
If you need to add 27.50ml of a solution, which piece of glassware would you use to deliver this volume and explain how you would determine if the 27.50 ml was measured?
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
A chemist prepares a solution of magnesium chloride MgCl2 by measuring out 49.mg of MgCl2 into a 100...
Questions


History, 03.12.2019 15:31

History, 03.12.2019 15:31



Mathematics, 03.12.2019 15:31

Mathematics, 03.12.2019 15:31






History, 03.12.2019 15:31


Biology, 03.12.2019 15:31



Mathematics, 03.12.2019 15:31


Mathematics, 03.12.2019 15:31

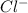 anions in the chemist's solution is 0.0104 M
anions in the chemist's solution is 0.0104 M
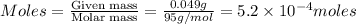
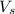 = volume of solution in ml = 100 ml
= volume of solution in ml = 100 ml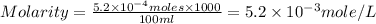
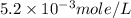
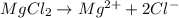
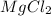 gives 2 moles of
gives 2 moles of 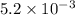 moles of
moles of