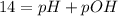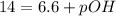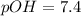Chemistry, 15.04.2020 00:57 sainijasdeep27
In pure water, some of the molecules ionize according to the equation H2O→H+ + OH−. The extent of the ionization increases with temperature. A student heats pure water and records the measured pH at 50°C as 6.6. Based on this information, what mathematical relationships gives the pOH pOH of pure water at 50°C?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

You know the right answer?
In pure water, some of the molecules ionize according to the equation H2O→H+ + OH−. The extent of th...
Questions

Biology, 19.05.2020 15:31


Mathematics, 19.05.2020 15:31

Mathematics, 19.05.2020 15:31



Social Studies, 19.05.2020 15:31


Mathematics, 19.05.2020 15:31

Computers and Technology, 19.05.2020 15:31

English, 19.05.2020 15:31



Chemistry, 19.05.2020 15:31

Mathematics, 19.05.2020 15:31


History, 19.05.2020 15:31


Mathematics, 19.05.2020 15:31

Mathematics, 19.05.2020 15:31

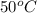 is,
is, 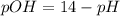
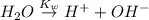
![K_w=[H^+][OH^-]](/tpl/images/0600/4070/bc68a.png)
![\log K_w=\log [H^+]+\log [OH^-]](/tpl/images/0600/4070/f0b12.png)
![-\log K_w=-\log [H^+]+(-\log [OH^-])](/tpl/images/0600/4070/00e23.png)
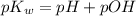
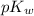 is 14 at 25-50°C.
is 14 at 25-50°C.