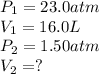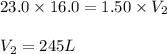Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1


Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2
You know the right answer?
A 16.0 16.0 L sample of neon gas has a pressure of 23.0 23.0 atm at a certain temperature. At the sa...
Questions


Mathematics, 22.01.2021 14:00

Physics, 22.01.2021 14:00

Health, 22.01.2021 14:00







Health, 22.01.2021 14:00

Arts, 22.01.2021 14:00

Mathematics, 22.01.2021 14:00


Chemistry, 22.01.2021 14:00



Mathematics, 22.01.2021 14:00


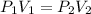
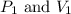 are initial pressure and volume.
are initial pressure and volume.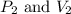 are final pressure and volume.
are final pressure and volume.