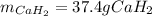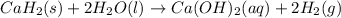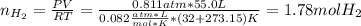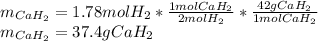Chemistry, 15.04.2020 00:28 swaggirllely36
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2 H2(g) Determine the number of grams of CaH2 are needed to generate 55.0 L of H2 gas at a pressure of 0.811 atm and a temperature of 32°C.\

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
Calcium hydride (CaH2) reacts with water to form hydrogen gas: CaH2(s) + 2 H2O(l) → Ca(OH)2(aq) + 2...
Questions

Business, 04.06.2021 17:40

Mathematics, 04.06.2021 17:40

History, 04.06.2021 17:40

Mathematics, 04.06.2021 17:40

Mathematics, 04.06.2021 17:40

History, 04.06.2021 17:40


Mathematics, 04.06.2021 17:40




Mathematics, 04.06.2021 17:40


Social Studies, 04.06.2021 17:40



History, 04.06.2021 17:40

Mathematics, 04.06.2021 17:40


Mathematics, 04.06.2021 17:40