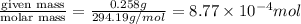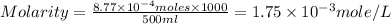Chemistry, 15.04.2020 00:18 sarinaneedshelp01
A chemist prepares a solution by adding 258 mg of K2Cr2O7 (MW = 294.19 g/mol ) to a volumetric flask, and then adding water until the total volume of the contents of the flask reaches the calibration line that indicates 500 mLmL. Determine the molarity of the prepared solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2
You know the right answer?
A chemist prepares a solution by adding 258 mg of K2Cr2O7 (MW = 294.19 g/mol ) to a volumetric flask...
Questions










Computers and Technology, 13.02.2020 18:16


Biology, 13.02.2020 18:17







Computers and Technology, 13.02.2020 18:17

Mathematics, 13.02.2020 18:17

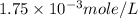
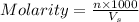
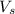 = volume of solution in ml = 500 ml
= volume of solution in ml = 500 ml