Chemistry, 15.04.2020 00:12 mariamsakayanthebest
A bomb calorimeter has a heat capacity of 783 J/oC and contains 254 g of water whose specific heat capacity is 4.184 J/goC. How much heat, in kilojoules, is evolved or absorbed by a reaction when the temperature goes from 23.73 oC to 26.01 oC? cH2O = 4.184 J/g. K

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
A bomb calorimeter has a heat capacity of 783 J/oC and contains 254 g of water whose specific heat c...
Questions



History, 21.11.2020 01:00


History, 21.11.2020 01:00






History, 21.11.2020 01:00


Law, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Mathematics, 21.11.2020 01:00

Health, 21.11.2020 01:00


Biology, 21.11.2020 01:00


![q=[q_1+q_2]](/tpl/images/0600/1829/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0600/1829/1d50b.png)
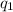 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter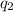 = heat absorbed by the water
= heat absorbed by the water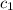 = specific heat of calorimeter =
= specific heat of calorimeter = 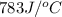
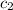 = specific heat of water =
= specific heat of water = 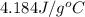
 = mass of water = 254 g
= mass of water = 254 g = change in temperature =
= change in temperature = 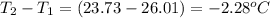
![q=[(783J/^oC\times -2.28^oC)+(254g\times 4.184J/g^oC\times -2.28^oC)]](/tpl/images/0600/1829/13e88.png)