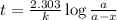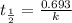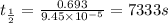Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Plz mark brainliest 30 points 1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s. 2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1


Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
The isomerization of methylisonitrile to acetonitrile CH3NC(g) → CH3CN(g) is first order in CH3NC. T...
Questions

Health, 05.11.2019 02:31

Social Studies, 05.11.2019 02:31


Mathematics, 05.11.2019 02:31










Social Studies, 05.11.2019 02:31


Mathematics, 05.11.2019 02:31


Mathematics, 05.11.2019 02:31


Mathematics, 05.11.2019 02:31