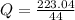Chemistry, 15.04.2020 02:11 espinosajoselyn
A 5.91 g of octane, C8H18, reacts with excess oxygen in a bomb calorimeter. The heat capacity of the calorimeter is 6.97 kJ/°C and the temperature increases by 32.0°C. How much heat, in units of kJ/mol, was absorbed by the bomb calorimeter?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3


Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
A 5.91 g of octane, C8H18, reacts with excess oxygen in a bomb calorimeter. The heat capacity of the...
Questions



Mathematics, 02.10.2019 09:50

Mathematics, 02.10.2019 09:50



Health, 02.10.2019 09:50

Biology, 02.10.2019 09:50



Mathematics, 02.10.2019 09:50

Advanced Placement (AP), 02.10.2019 09:50






Mathematics, 02.10.2019 09:50


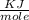
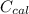 = 6.97
= 6.97 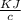
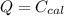 ΔT
ΔT