

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 23.06.2019 06:30
Acompound has the molecular formula c3h8. which class of organic compounds does it belong to?
Answers: 2

You know the right answer?
Lithium and nitrogen react in a combination reaction to produce lithium nitride: 6Li(s) + N2(g) → 2L...
Questions

Mathematics, 18.03.2021 01:00





Mathematics, 18.03.2021 01:00

Physics, 18.03.2021 01:00



Mathematics, 18.03.2021 01:00



Chemistry, 18.03.2021 01:00

Spanish, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00

Mathematics, 18.03.2021 01:00

Advanced Placement (AP), 18.03.2021 01:00


Mathematics, 18.03.2021 01:00

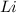 are needed to produce 0.45 mol of
are needed to produce 0.45 mol of 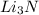
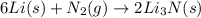
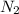 is the excess reagent.
is the excess reagent.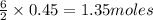 of
of 

