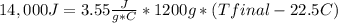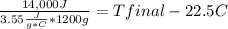Chemistry, 14.04.2020 22:50 emmaraeschool
Complete combustion of a 0.350 g sample of a compound in a bomb calorimeter releases 14.0 kJ of heat. The bomb calorimeter has a mass of 1.20 kg and a specific heat of 3.55 J/(gi°C). If the initial temperature of the calorimeter is 22.5°C, what is its final temperature? Use q equals m C subscript p Delta T..

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Describe the difference between a. a hypothesis and a theory and b. an observation and an experiment.
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

You know the right answer?
Complete combustion of a 0.350 g sample of a compound in a bomb calorimeter releases 14.0 kJ of heat...
Questions

Advanced Placement (AP), 17.07.2020 01:01


Mathematics, 17.07.2020 01:01



Physics, 17.07.2020 01:01

English, 17.07.2020 01:01




Mathematics, 17.07.2020 01:01



English, 17.07.2020 01:01





History, 17.07.2020 01:01

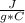 m=1.20 kg= 1200 g (1 kg=1000 g)Tfinal= ?Tinitial= 22.5 °C
m=1.20 kg= 1200 g (1 kg=1000 g)Tfinal= ?Tinitial= 22.5 °C