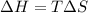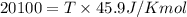Chemistry, 14.04.2020 22:27 laylac45531
For a particular reaction, Δ H ∘ = 20.1 kJ/mol and Δ S ∘ = 45.9 J / (mol ⋅ K). Assuming these values change very little with temperature,
at what temperature does the reaction change from nonspontaneous to spontaneous in the forward direction? T = K

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Subduction zones occur on earth where dense oceanic crust dives under more buoyant continental crust. these boundaries are characterized by a deep ocean trench next to a high continental mountain range, large numbers of earthquakes and volcanoes. all of this is further evidence for the a) big bang theory b) origin of the species eliminate c theory of plate tectonics d theory of natural selection 4 sedimentary rocks found high in the himalayen mountain
Answers: 1

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1
You know the right answer?
For a particular reaction, Δ H ∘ = 20.1 kJ/mol and Δ S ∘ = 45.9 J / (mol ⋅ K). Assuming these values...
Questions




Mathematics, 30.09.2019 11:30


History, 30.09.2019 11:30

History, 30.09.2019 11:30

History, 30.09.2019 11:30

Business, 30.09.2019 11:30



Spanish, 30.09.2019 11:30


Mathematics, 30.09.2019 11:30


English, 30.09.2019 11:30




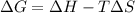
 = Gibb's free energy change
= Gibb's free energy change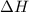 = enthalpy change = 20.1 kJ/mol = 20100 J/mol
= enthalpy change = 20.1 kJ/mol = 20100 J/mol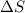 = entropy change = 45.9 J/Kmol
= entropy change = 45.9 J/Kmol