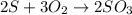Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which of the following is a compound? a.carbon b.oxygen c.hydrogen d.water
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
60.0 g O2 and 50.0 g S are reacted according to the equation 2 S + 3 O2 → 2 SO3 . Which reactant is...
Questions


Mathematics, 03.04.2020 03:07

English, 03.04.2020 03:07

Mathematics, 03.04.2020 03:07

Mathematics, 03.04.2020 03:07

Social Studies, 03.04.2020 03:07

Mathematics, 03.04.2020 03:08

Social Studies, 03.04.2020 03:08




Mathematics, 03.04.2020 03:08


Mathematics, 03.04.2020 03:08


Biology, 03.04.2020 03:08




Physics, 03.04.2020 03:08